Hydrogen Generating Plant by Water Electrolysis
View More+-
-
Hydrogen Generator by Natural Gas Steam Reforming
View More+ -

Hydrogen Generation Plant by Methanol
View More+ -

Hydrogen Generator by Ammonia Decomposition with Purifying System
View More+ -

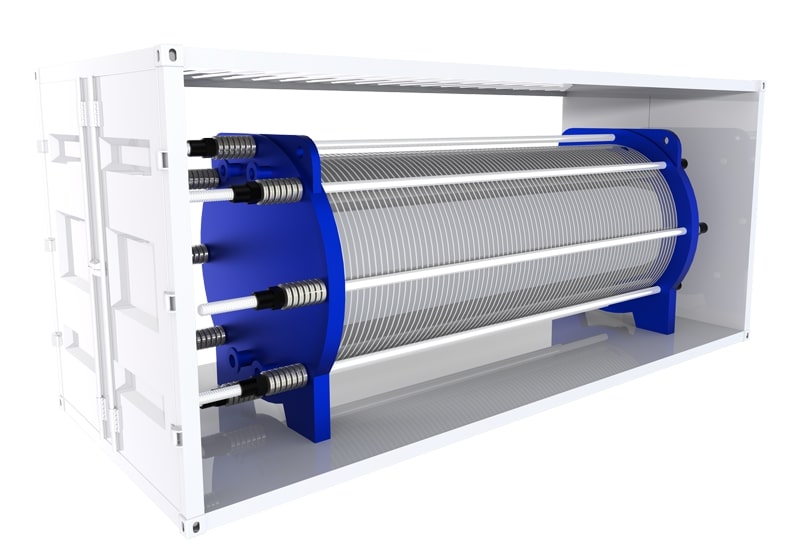
Pressure Swing Adsorption Hydrogen Generator with Purifying System
View More+
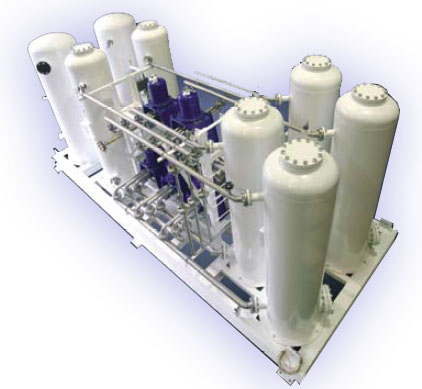
Hydrogen Plant by Pressure Swing Adsorption with Purifying System
Inquiry and Sales: sales@angstrom-advanced.com or Online Request

Pressure Swing Adsorption(PSA) is used to separate hydrogen gas from other gases by feeding the gas under certain pressure. This separation process
occurs in adsorption tower. The main advantages behind this technology is its low cost, low capacity, minimal maintenance requirements and a
user-friendly interface.
The H2 Purifying System removes oxygen from Hydrogen via a catalyst to get high purity H2 after adsorption of drying through the dust filter.
The system can remove oxygen levels down to 1ppm. Through one step of purification, the purity can reach to 99.999~99.9999% and dew point can
be lower than -70°C; Through a second purification step, O2 can be lowered down to less than 0.1ppm and dew point can be lowered to -90°C.
Pressure-Swing Adsorption or PSA is a technical process widely used these days in gas production. It's a typically non-cryogenic way of purifying gas. PSA process differs from cryogenic techniques of gas separation as it can function under near-ambient temperatures. Under pressure, the PSA process separates some 'species' from a mixture, according to the molecular characteristics of those 'species'. This is because different gases has the propensity to be attracted to different solid surfaces more or less strongly.
More gas is adsorbed at higher pressure while the gas is desorbed as the pressure is reduced. Suppose air under pressure is passed through a vessel containing an adsorbent bed and this bed has the property to attract nitrogen more strongly than oxygen. What will happen is that a part or all of the nitrogen will remain in the bed, while the gas coming out of the vessel will be enriched in oxygen. As the bed reaches its optimum capacity to adsorb nitrogen, it can again be regenerated by reducing the pressure. Releasing the adsorbed nitrogen in the process. Thus readying itself for another cycle of oxygen enriched air. This cycle goes on.
Popular adsorptive materials like carbon, zeolites, silica etc. are used as the adsorbent beds. They selectively adsorbs the undesired gases at high pressure on their surface. Continuous production of the target gas is achieved by using more than one adsorbent chamber. However it is not a very easy process and difficult to model accurately. But once properly modeled, adsorption process can result in complex diffusion relationships.
| H2 capacity | 10 ~ 5000 Nm3/h |
| Dew Point | ≤ -60℃ |
| H2 purity | 98 ~ 99.999% |
| Work pressure | 0.2 ~ 4.0 MPa |
